cadmium electron configuration|Cadmium Electron Configuration (Cd) with Orbital : Tagatay Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa DILG Secretary Benjamin Abalos Jr. (File photo) MANILA – Department of the Interior and Local Government (DILG) Secretary Benjamin "Benhur" Abalos Jr. called on the agency's 49 new lawyers to continue working together to build a country that embraces fairness, justice, and inclusivity.. In a statement Thursday, Abalos congratulated the .4. The &near= Search Parameter. There is a URL parameter you can append to your Google search to return results near a certain location — just add &near=cityname to your query string, where .
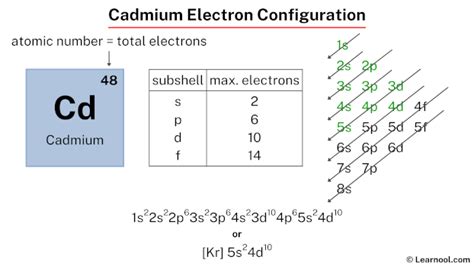
cadmium electron configuration,The ground-state electron configuration of cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. This electron configuration shows that the last shell of cadmium has two electrons and the d-orbital has a total of ten electrons. Therefore, the valence electronsof cadmium are two. The . Tingnan ang higit pacadmium electron configurationThe total number of electrons in cadmium is forty-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit pa Learn how to write the electron configuration of cadmium using the aufbau principle, periodic table, Bohr model, or orbital diagram. See the steps, formulas, and examples for each method.Learn the electron configuration of cadmium, a transition metal with atomic number 48 and symbol Cd. Find out its properties, uses, sources and effects on the environment and food.
Learn about the electron configuration, isotopes, metallic characteristics, natural occurrences, and common reactions of cadmium, a toxic transition metal. Find .Cadmium Electron Configuration (Cd) with Orbital When we write the configuration, we'll put all 48 electrons in orbitals around the nucleus of the Cadmium atom. In this video we'll use the Periodic Table to .
What is the Electron Configuration of Cadmium? Kr 4d10 5s2 is the electron configuration of Cadmium. How Many Valence Electrons Does Cadmium Have. There are two valence electrons in the outer shell .

Cadmium electron configuration. ← Electronic configurations of elements. Cd (Cadmium) is an element with position number 48 in the periodic table. Located in the V period. .Cadmium is a silvery metal with a bluish tinge and an electron configuration of [Kr] 4d 10 5s 2. It is toxic, carcinogenic and teratogenic, and is used in batteries, coatings, pigments .Cadmium is a transition metal with symbol Cd and atomic number 48. Its electron configuration is [Kr] 4d 10 5s 2, with two valence electrons. Learn more about its . Learn about the electron configuration of cadmium, a transition metal with the symbol Cd and atomic number 48. Find out how cadmium is used, where it is found, and .Chemical element, Cadmium, information from authoritative sources. Look up properties, history, uses, and more. . 2.2 Electron Configuration [Kr]5s 2 4d 10. Los Alamos National Laboratory, U.S. Department of .Element Cadmium (Cd), Group 12, Atomic Number 48, d-block, Mass 112.414. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the .
Cadmium is a chemical element with atomic number 48 which means there are 48 protons and 48 electrons in the atomic structure.The chemical symbol for Cadmium is Cd. Electron Configuration and Oxidation States of Cadmium. Electron configuration of Cadmium is [Kr] 4d10 5s2. Possible oxidation states are +2. Electron . The normal ground state electronic configuration of Cadmium element is Cd: [Kr] 4d 10 5s 2. But when it loses 2 electrons, its electronic configuration becomes Cd +2: [Kr] 4d 10.In this state, if we see the electron configuration of Cadmium, then the d-orbitals are completely filled (it has complete 10 electrons).Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2; Electrons per Energy Level: 2,8,18,18,2 Shell Model; . Cadmium - Cd (EnvironmentalChemistry.com)- Comprehensive information for the element Cadmium - Cd is provided by this page including scores of properties, element names in many .

Symbol: Cd Date of discovery: 1817 Name origin: Greek kadmeia Appearance: silvery Discoverer: Fredrich Stromeyer Obtained from: by-product, zinc refining Melting point: 320.9 K Boiling point: 765 K Density[kg/m 3]: 8.65 Molar volume: 13.00 × 10-6 m 3 /mol Protons/Electrons: 48 Neutrons: 64 Shell structure: 2,8,18,18,2 Electron . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
cadmium electron configuration Cadmium Electron Configuration (Cd) with Orbital Abbreviated electronic configuration of Cadmium. The ground state abbreviated electronic configuration of Neutral Cadmium atom is [Kr] 4d10 5s2. The portion of Cadmium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Kr]. For atoms with many electrons, this notation can become lengthy .Electronic configuration of the Cadmium atom. Valence electrons. Orbital diagram. Cadmium electron configuration. ← Electronic configurations of elements . Cd (Cadmium) is an element with position number 48 in the periodic table. Located in the V period. Melting point: 321 ℃.
Electron configuration 4d 10 5s 2: Electrons per shell: 2, 8, 18, 18, 2: Physical properties; . Cadmium is a chemical element; it has symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.Cadmium, complete electron configuration. © 2009-2016 | www.prvky.com | kontaktkontakt4d–series consists of elements from Y (atomic number 39) to Cd (atomic number 48). These elements lie in the 5 th period of the periodic table.In this series, the differentiating electron occupies 4d orbitals, i.e., the elements of this series involve the progressive filling of 4d orbitals as we proceed from Y 39 to Cd 48.La configuration électronique du cadmium est 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. L'élément chimique situé dans le groupe 12 du tableau périodique est appelé cadmium, son numéro atomique est 48 et le symbole de cet élément est Cd. Basic Steps. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Subshells are described by writing the principal quantum number n followed by the symbol for the angular momentum quantum number l (s, p, d, or f). The the total number of electrons in each subshell is written as a .La configurazione elettronica del cadmio è 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. L'elemento chimico situato nel gruppo 12 della tavola periodica è chiamato cadmio, il suo numero atomico è 48 e il simbolo di questo elemento è Cd.
cadmium electron configuration|Cadmium Electron Configuration (Cd) with Orbital
PH0 · How to Write the Electron Configuration for Cd and Cd2+
PH1 · Electron configuration of Cadmium
PH2 · Electron configuration for Cadmium (element 48). Orbital diagram
PH3 · Complete Electron Configuration for Cadmium (Cd, Cd2+)
PH4 · Chemistry of Cadmium
PH5 · Cadmium electron configuration
PH6 · Cadmium Electron Configuration (Cd) with Orbital
PH7 · Cadmium (Cd)
PH8 · Cadmium
PH9 · 11.2: Chemistry of Cadmium